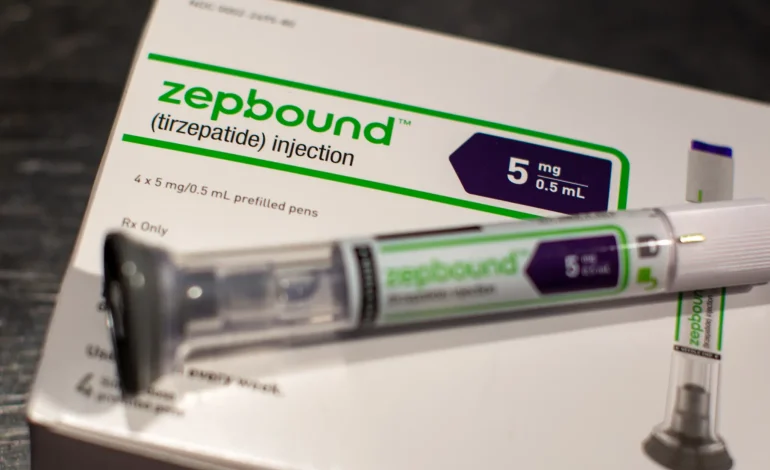
On December 20, the US Food and Drug Administration (FDA) approved Zepbound (tirzepatide), developed by Eli Lilly, as the first medication to treat obstructive sleep apnea (OSA) in adults with obesity, New York Post reports.
This marks a significant advancement in the treatment options available for those suffering from the condition.
Obstructive sleep apnea occurs when the upper airway becomes blocked during sleep, leading to pauses in breathing. It is particularly common in individuals who are overweight or obese. Traditionally, the most common treatment for OSA has been positive airway pressure (PAP) therapy. However, many patients struggle with adherence to this treatment, highlighting the need for alternative or adjunctive options.
Zepbound is intended to be used alongside a reduced-calorie diet and increased physical activity, as part of a comprehensive approach to managing OSA. The drug works by activating hormones in the intestine (GLP-1 and GIP), which help reduce appetite and food intake, similar to treatments like semaglutide (Ozempic, Wegovy).
In clinical trials, Zepbound showed promising results for both weight loss and improvements in sleep apnea symptoms. In a 52-week study, participants treated with Zepbound experienced a statistically significant reduction in the number of apnea and hypopnea events. A significant portion of participants also reported achieving remission or a resolution of symptoms. Along with these improvements, patients also saw a notable decrease in body weight.
Sally Seymour, M.D., director of the FDA’s Division of Pulmonology, Allergy, and Critical Care, praised the approval, calling it a major step forward for patients with OSA. She emphasized that this is the first drug treatment option available for certain patients with the condition.
While Zepbound offers potential benefits, it also comes with some side effects. Patients may experience nausea, diarrhea, vomiting, constipation, stomach discomfort, and fatigue, among others. The drug has also been shown to cause thyroid C-cell tumors in rats, although its effects in humans are still unknown. As a precaution, the FDA advises against using Zepbound in patients with a history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2.
The FDA urges patients to consult with a doctor before starting treatment and to monitor for any adverse effects.
Sleep expert Dr. Wendy Troxel, a senior behavioral specialist at the RAND Corporation, described the approval as a “promising advancement” for the millions of people affected by sleep apnea. She highlighted that, while PAP therapy is highly effective, many patients are non-adherent, making this new medication an important option for those who struggle with existing treatments.
Dr. William Lu, medical director of Dreem Health, also commented on the significance of Zepbound, noting that obesity and sleep apnea are both prevalent health conditions in the US. He called Zepbound a potential “generational medication” that could improve patients’ overall health by addressing both issues simultaneously.