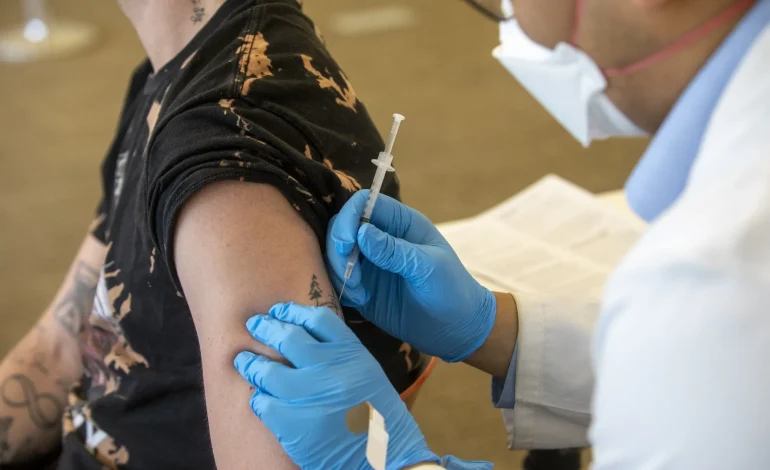
A new study conducted in Ontario, Canada, suggests that Bavarian Nordic A/S’s smallpox vaccine provides moderate protection against mpox infection after a single dose, Bloomberg reports.
The findings, published in The BMJ, mark the vaccine’s potential role in curbing the spread of the virus, particularly in high-risk populations.
The study, conducted from mid-June to late October 2022, focused on gay and bisexual men, the group most affected by the 2022 mpox outbreak. Researchers found that a single dose of the MVA-BN vaccine (Imvamune in Canada, Jynneos in the US) provided around 58% protection against mpox infection.
The study comes at a critical time, as health experts are working to understand the effectiveness of existing vaccines against a potentially more dangerous clade Ib monkeypox virus strain circulating in central Africa. The current vaccines, originally developed for smallpox, have not been evaluated in randomized, controlled trials for mpox, making real-world data crucial for assessing their efficacy.
“In the absence of randomized clinical trials, our findings strengthen the evidence that MVA-BN is effective at preventing mpox infection and should be made available and accessible to communities at risk,” stated scientists from the Canadian Immunization Research Network Provincial Collaborative Network.
While the study found moderate effectiveness for a single dose, the researchers emphasize the importance of achieving high coverage with a full two-dose regimen to prevent and manage ongoing transmission globally. This is particularly relevant as the emergence of the clade Ib strain has sparked a global health emergency.
While Canada initially employed a dose-sparing strategy due to supply concerns, a two-dose regimen was implemented on September 30, 2022. However, the study period ended before a significant number of individuals received the second dose, limiting the researchers’ ability to assess its effectiveness.